Trump Administration’s Drug Rebate Program Blocked by Court: that’s the headline echoing across healthcare policy circles, hospital boards, pharmacy chains, and even community clinics in small towns. In January 2026, a federal appeals court slammed the brakes on a major shift to how hospitals and health centers serving low-income communities would pay for prescription drugs under the 340B Drug Pricing Program. This wasn’t just any paperwork shuffle — it had real consequences for how millions of Americans access affordable medications. So, what’s all the fuss about? Why was this rebate program so controversial? And what should patients, insurers, hospitals, and policymakers take away from the ruling? Let’s break it all down.
Table of Contents
Drug Rebate Program
The Trump Administration’s attempt to overhaul the 340B Drug Pricing Program through a rebate model has been blocked — at least for now — by the courts. While drug manufacturers advocated for more streamlined oversight, hospitals raised valid concerns about financial sustainability and patient access. The result? The court sided with caution. For patients, nothing changes today. But for policymakers, this is a clear signal that healthcare reform must be handled transparently, collaboratively, and with a strong grasp of the real-world impacts on people and providers.

| Topic | Details |
|---|---|
| Policy Blocked | 340B Rebate Model Pilot Program proposed by the Trump Administration |
| Who Blocked It | U.S. First Circuit Court of Appeals |
| Reason for Block | Program lacked proper analysis, risked harm to hospitals serving vulnerable populations |
| Affected Group | Safety-net hospitals, community clinics, low-income patients |
| Current Status | Program is paused; legal review ongoing |
| Agency Involved | Health Resources and Services Administration (HRSA) – hrsa.gov |
| Opposition | American Hospital Association, rural hospital networks, major health systems |
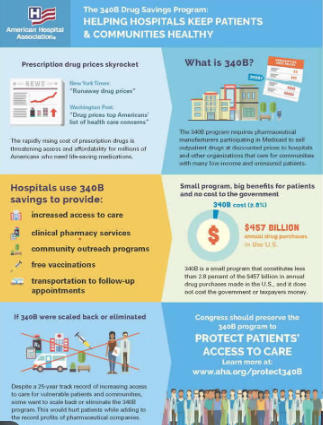
What Is the 340B Drug Pricing Program?
First things first: to understand what got blocked, you need to understand what was being changed.
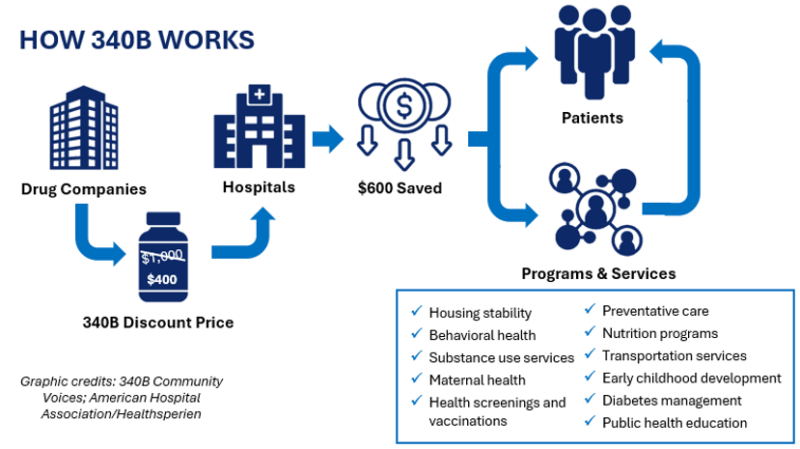
The 340B Drug Pricing Program, established in 1992, requires pharmaceutical companies to provide outpatient medications at reduced prices to eligible healthcare organizations. These include hospitals, health centers, and clinics that serve low-income or rural populations.
Here’s the key: under 340B, discounts are applied upfront. That means when a qualifying hospital buys insulin, chemotherapy drugs, or asthma inhalers, they pay a lower price at the time of purchase. That discount helps the hospital keep services running for those who often can’t pay out-of-pocket.
Over the years, the program has grown significantly. As of 2025, over 50,000 sites across the U.S. were enrolled, and the program accounted for more than $66 billion in outpatient drug purchases annually
What Did the Trump Administration Propose For Drug Rebate Program?
The Trump Administration’s Health Resources and Services Administration (HRSA) proposed what it called the 340B Rebate Model Pilot Program. This wasn’t a law, but an administrative policy shift introduced in mid-2025.
Instead of drug discounts being applied at the time of purchase, HRSA’s new model would have worked like this:
- Hospitals would pay full price for medications initially.
- Drug manufacturers would issue rebates later.
Why make this change?
The official rationale was to reduce duplicate discounts, tighten tracking of discount eligibility, and bring rebate accounting in line with Medicaid best practices. Some officials believed it would also give the government better oversight into how the 340B discounts were used by hospitals.
Pharmaceutical companies largely supported the change. From their perspective, the rebate model would streamline operations and reduce the chance of overlapping government discounts — like when both Medicaid and 340B apply to the same drug.
But hospitals saw it differently.
Why Did Hospitals Push Back?
The strongest opposition came from the American Hospital Association (AHA), along with dozens of regional hospital groups and public health coalitions.
Their argument was simple: this change would create severe financial strain on facilities that are already running on razor-thin budgets.
Let’s look at some real-world challenges:
- A community hospital in rural Nebraska that currently pays $50 for a vial of insulin under the 340B program would now have to front $300 per vial and wait weeks — maybe months — for the rebate.
- Hospitals feared the change would lead to service cutbacks, staff layoffs, or even closures in vulnerable areas.
Hospitals also pointed out that HRSA bypassed normal rulemaking procedures, failing to conduct a full economic impact analysis and not allowing sufficient time for stakeholder feedback. In legal terms, they accused HRSA of violating the Administrative Procedure Act (APA).
In December 2025, a federal judge in Maine granted a preliminary injunction, halting the program before it could take effect in January 2026. Then, in January 2026, the U.S. First Circuit Court of Appeals upheld that injunction, effectively freezing the program.
What Did the Court Say About Drug Rebate Program?
In its decision, the court made three major points:
- Procedural Missteps: HRSA likely failed to follow proper rulemaking procedures required under the APA. That’s a big deal in administrative law.
- Financial Harm: The court agreed that hospitals could face “irreparable harm” if the policy were implemented without proper planning.
- Lack of Impact Analysis: HRSA did not provide convincing evidence that it analyzed the program’s effects on hospital finances or patient care delivery.
The court did not rule on the legality of the program itself — only that it cannot be implemented without a more thorough and lawful process.
What Happens Now?
For now, the status quo remains. Hospitals will continue to receive discounted drug prices upfront, and the pilot rebate program is on hold indefinitely.
Insiders in Washington suggest that the Trump Administration may withdraw its appeal entirely, particularly after the strong reaction from healthcare providers and legal scholars.
However, the bigger issue — how to modernize and regulate the 340B program — is far from over.
Perspectives from Stakeholders
Hospital Executives
“We’re not opposed to oversight. But making us float drug costs for months would devastate our operating margins,” said Julie Doyle, CFO of a nonprofit hospital in Missouri.
Pharmaceutical Industry
“There are real compliance issues with the current system,” said an executive from a major U.S. drug manufacturer. “The rebate model gives us better visibility and audit trails. It’s not just about money — it’s about integrity.”
Public Health Experts
Many policy experts agree reform is needed but question the sudden nature of HRSA’s rollout. “Reform should be collaborative and data-driven,” noted Dr. Kevin Ryan, a public health professor at Emory University. “Doing it right is more important than doing it fast.”
Broader Context: Is Reform Still Coming?
Yes — and here’s why:
- A 2022 GAO report found inconsistencies in how hospitals tracked and reported 340B savings.
- Bipartisan members of Congress have introduced legislation to increase transparency without disrupting hospital finances.
- Drug spending under Medicare continues to rise, putting pressure on lawmakers to seek accountability across all pricing systems — including 340B.
Even if the rebate model is off the table for now, reform of some kind is likely inevitable.
What Patients and Insurers Should Know?
For Patients:
- You won’t see changes at your pharmacy as a result of this court ruling.
- If you get care at a 340B-eligible facility, your access to affordable medications is protected — for now.
- Keep in mind: if major reforms are rushed in the future, they could impact care delivery at hospitals in underserved areas.
For Insurers:
- The rebate model would not have changed plan premiums or reimbursement directly.
- However, large-scale changes to how hospitals purchase drugs could indirectly affect provider contracts and drug formulary structures in the future.
- Payers should stay informed on legislative reform and potential downstream effects on claims pricing and network participation.
Social Security Digital Payments – Why 500,000 Seniors Must Switch From Paper Checks This Fall
$1000 Trump Stimulus Check 2026: Who Could Qualify and When Payments Might Arrive
Social Security Fairness Act Update – When Back Payments Begin and What Monthly Amounts Change